Fathomers Dispatch 01
What Is Synthetic Biology?
When you hear the words “synthetic biology,” what do you think of? GMO tomatoes, or lab-grown meat? Or perhaps you read in Science News about how yeast can now be genetically programmed to create artemisinin, an antimalarial drug normally harvested from the wormwood plant; or that scientists in the Netherlands developed tear duct gland “organoids” – miniature, simplified versions of organs – that can cry, no human superstructure needed; or maybe you came across a news article on the first xenobot, a synthetic, programmable organism designed by AI and derived from frog DNA, which in 2021 was able to self-replicate just by gathering and shaping loose cells in the environment.
Today, synthetic biology has no single, globally agreed-upon definition. In the broadest sense, “synbio” is a multidisciplinary new field of biotechnology that involves engineering the genetic material of organisms – such as bacteria, yeast, plants, or animals, including humans – to have new characteristics. At the nexus of biology and engineering, synthetic biology is set to become one of the most powerful technologies of this century.
Pictured: Lacrimal gland organoids under a simple microscope. Credit: Dr. Marie Bannier-Hélaouët, copyright ©: 2021 Hubrecht Institute.
There are basically two subfields: one seeks to reproduce structures from natural biology with non-natural molecules in order to create artificial life (for example: building a functional living cell from scratch), and the other seeks interchangeable parts from natural biology to assemble into systems that act unnaturally (for example: genetically modifying the bacteria e. coli to turn it into a tiny factory for producing novel antibiotics).
Thanks to recent technological breakthroughs, it's more possible than ever to become a synthetic biologist from the comfort of your own garage. For one thing, the rapid improvement in AI, including machine learning, has aided researchers in sorting through massive amounts of genetic data to find the most promising recombinations. CRISPR-Cas9, a novel gene editing tool, is also inexpensive, easy to use, and widely available. This technology utilizes a guide RNA (gRNA or CRISPR RNA/cRNA) to target a specific spot in the genome of a cell; when the target gene is found, the enzyme Cas9 acts as a pair of molecular scissors, cutting the two strands of DNA. The cell then recognizes that the DNA is damaged and tries to repair it, a process that can be manipulated by scientists to introduce desired changes to the genome. Simple CRISPR-Cas9 protocols are widely available online, and specialized kits can cost as little as USD $150.
Grassroots, “DIY-Bio” community labs can be found today in almost every major city, where enthusiasts conduct their own experiments far from university, federal, or corporate institutions. The diverse, flexible organization and open framework of these labs is reminiscent of the early days of computer coding in more ways than one. DNA is a sequentially coded language, too; we might one day teach DNA literacy the same way current high school students have the option to take coding classes.
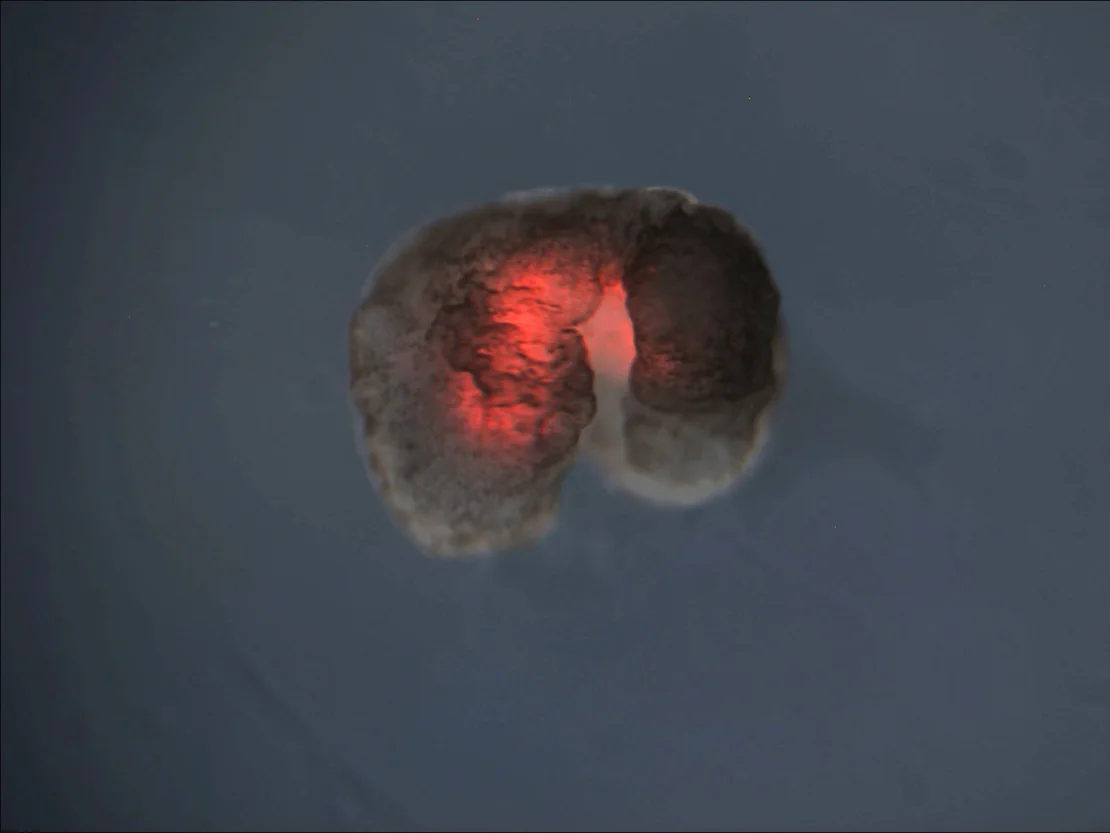
Pictured: A xenobot with large hind limbs and smaller forelimbs, layered with red heart muscle. Credit: Douglas Blackiston, Sam Kriegman
The possible applications for synthetic biology are incredibly various. Microbes are being engineered to decontaminate wastewater and harness carbon emissions; algae can be programmed to glow in the presence of chemical pollutants, or used to synthesize alternatives to animal-derived goods like horseshoe blood or omega-3 fish oils. Some have proposed releasing genetically engineered organisms into the environment to eradicate vectors of diseases, fight back against invasive species, and lend resilience to threatened plants and animals. Theoretically, it’s becoming possible to permanently “delete” some genetically inherited illnesses like sickle-cell disease, while synthetic organs might one day replace the need for organ donation.
As promising as they are, these applications pose significant ethical questions. “Misuse of these technologies and a failure to account for unintended consequences could cause irreversible environmental damage,” says specialist Pinya Sarasas, in a release from the United Nations Environment Programme. The kind of DNA editing conducted by synthetic biologists has the capacity to permanently alter our planet’s gene pool, since it’s now possible to make “heritable” changes that pass through multiple generations. It takes human meddling in the natural world to a whole new scale – a scale of centuries, even millenia. Dr. Frankenstein’s necromantic experimentations could come to look downright tame in comparison.
Pictured: Slices of mini–brain organoids with neural stem cells (red) and cortical neurons (green). Credit: Hajime Ozaki, Watanabe lab/UCI
We must ask each other: What forms of life will we choose to cultivate? What will we nurture, and what might we leave behind, whether by choice or by accident? Where does agency reside in this new moment of making, learning, and living with?
These questions are at the core of Emergence: A Genealogy, an exhibition that will open in September 2024 as part of the Getty PST ART: Art & Science Collide initiative. Organized across the themes “Being Born,” “Living,” and “Dying,” Emergence probes changing definitions of what is natural, synthetic, conscious, and essential to existence. We warmly invite you to join us. (Natasha Boyd, Fathomers Research Assistant, 2023)